
The end-stage aggregates present in these various diseases are β-sheet-rich and fibrillar in nature, but have distinct ultrastructures.

In Parkinson’s disease (PD) and dementia with Lewy bodies (DLB), α-synuclein (αSyn) accumulates within neurons as Lewy bodies and Lewy neurites. Tau aggregates are also found in progressive supranuclear palsy (PSP), corticobasal degeneration (CBD) and Pick’s disease (PiD). Alzheimer’s disease (AD) is characterized by two types of protein deposits: plaques composed of amyloid β-protein (Aβ) and tau-containing neurofibrillary tangles. The presence of macroscopic protein aggregates is pathognomonic for most common neurodegenerative disorders. These results indicate that PrP is likely to play an important role in a variety of late-life neurodegenerative diseases and that therapeutic targeting of PrP, rather than individual disease proteins, may have more benefit for conditions which involve the aggregation of more than one protein. Using an all-human experimental paradigm involving: (1) isogenic iPSC-derived neurons expressing or lacking PRNP, and (2) aqueous extracts from brains of individuals who died with Alzheimer’s disease, dementia with Lewy bodies, and Pick’s disease, we demonstrate that Aβ, α-synuclein and tau are toxic to neurons in a manner that requires PrP C. Moreover, soluble aggregates of tau, α-synuclein and Aβ cause both functional (impairment of LTP) and structural (neuritic dystrophy) compromise and these deficits are absent when PrP is ablated, knocked-down, or when neurons are pre-treated with anti-PrP blocking antibodies. Here, we show that soluble aggregates of α-synuclein and tau bind to plate-immobilized PrP in vitro and on mouse cortical neurons, and that this binding requires at least one of the same N-terminal sites at which soluble Aβ aggregates bind. Nearly all of these diseases are characterized by oligomerization and fibrillization of neuronal proteins, and there is great interest in therapeutic targeting of these aggregates. Our observations indicate that realistic complex mixtures of environmental pollutants can affect neuronal connectivity via NGF-induced neurite outgrowth.Neurodegenerative diseases are an enormous public health problem, affecting tens of millions of people worldwide. Neither the POP mixture nor PFOS affected neurite length in the absence of NGF. PFOS induced neurite outgrowth to about 50 % of the level seen with the POP mixture. The pollutants did not inhibit neuritogenesis, but rather increased NGF-induced neurite length. Live imaging, using the IncuCyte ZOOM platform indicated ongoing cell proliferation over time, but slower in presence of NGF. Robust glutathione levels were observed in NGF-differentiated cells. High-content analysis showed a decrease in cell number, but no changes for nuclear and mitochondrial cellular health parameters.
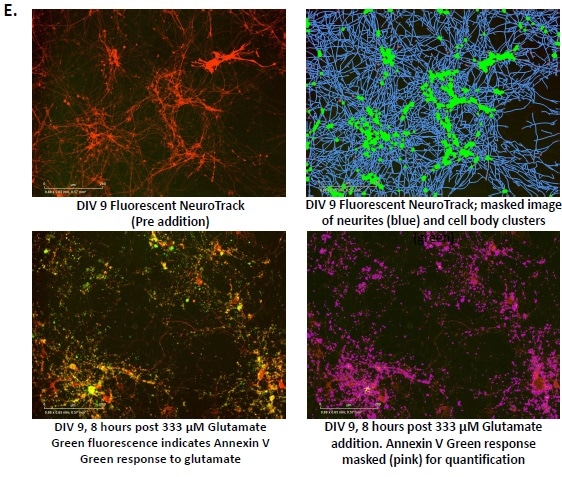
Only higher concentrations of POP mixture reduced tetrazolium salt (MTT) conversion. We also evaluated perfluorooctane sulfonic acid (PFOS) alone, the most abundant compound in the POP mixture.

Cells were exposed for 72 h to a defined mixture of POPs with chemical composition and concentrations based on blood levels in the Scandinavian population.
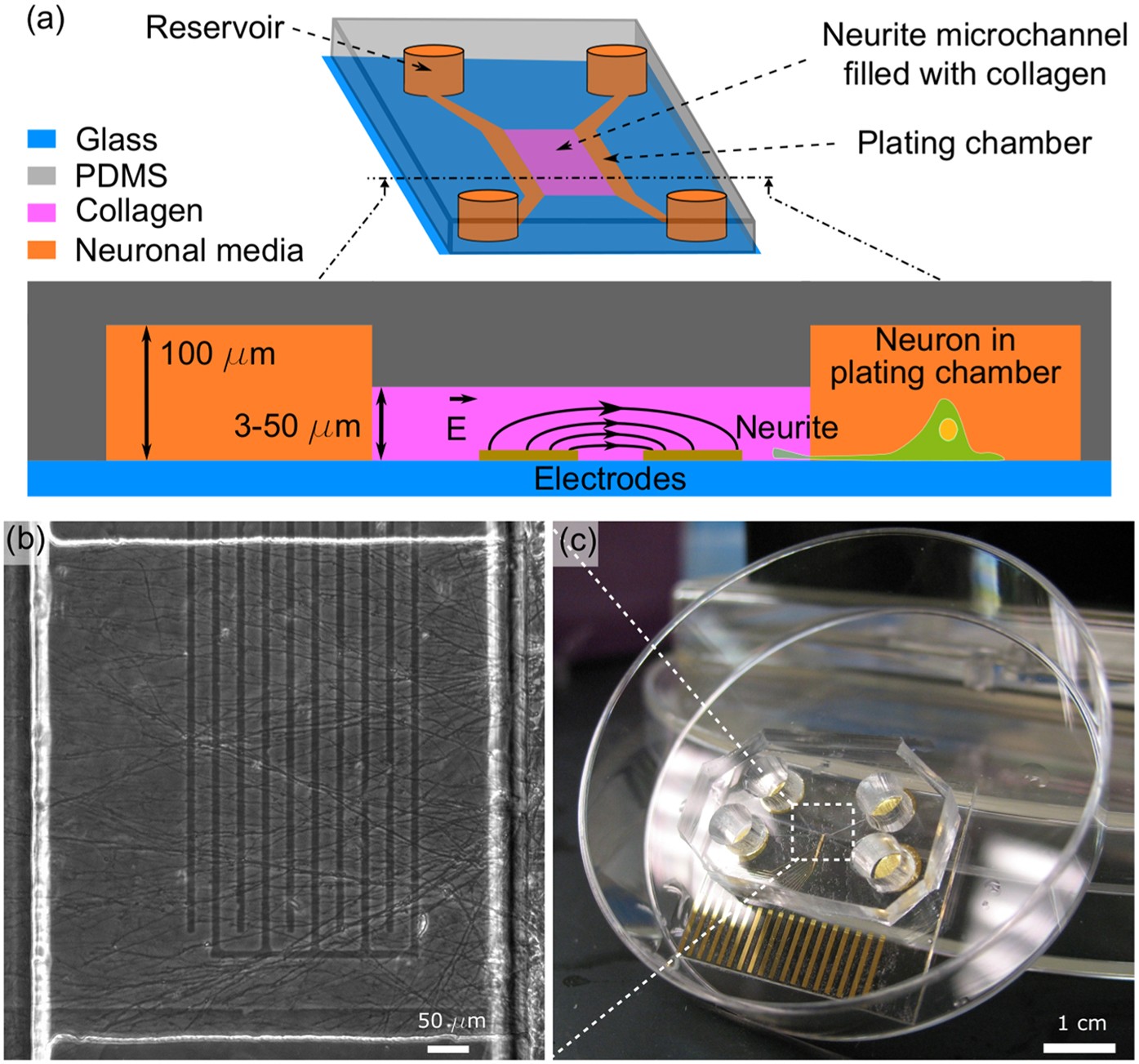
We investigated their effect on neurite outgrowth in PC12 rat pheochromocytoma cells, in absence or presence of nerve growth factor (NGF), an inducer of neuronal differentiation. Persistent organic pollutants (POPs) are potential developmental neurotoxicants. Disruption of neurite outgrowth is a marker for neurotoxicity.


 0 kommentar(er)
0 kommentar(er)
